Vascular Network-mimetic Oxygen-Transporting Mesh for Islet Graft
Contact PI: Hirotake Komatsu, PhD, City of Hope (R03 DK129958)
Start Date: July 1, 2021
End Date: June 30, 2023
NIH HIRN Gateway Investigator Award Recipient
Abstract
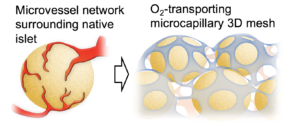
Patients with type 1 diabetes (T1D) benefit from cell replacement therapy using insulin-producing pancreatic islet cells, which are typically sourced from deceased donors. To overcome an existing shortage of cadaveric islets, stem cell-derived beta cells are rapidly emerging as a promising alternative source. However, stem cell-derived beta cells require close monitoring and retrievability; to date, the subcutaneous (SC) tissue is the only site available to accommodate these requirements. However, the SC site faces a major challenge in achieving an adequate oxygen (O2) supply. Lack of an appropriate SC transplantation platform, due to the failure to overcome hypoxia, hinders both research progress and clinical translation of stem cell-derived beta cells. Without achieving effective engraftment in the SC site, the overall strategy of beta cell replacement therapy will not be successful. In alignment with the mission of the Human Islet Research Network (HIRN) NIDDK consortium to find innovative strategies to protect or replace functional beta cell mass in people with T1D, my group proposes to transform the hypoxic SC site into an oxygenated site using an innovative microdevice. The overall device is a thin (25 µm-thick) and flexible O2-transporting 3D mesh. Our microdevice is distinct from other existing oxygenation devices in several innovative aspects: 1) it uses a biomimetic, vascular network-like structure of synthetic microcapillaries to transport and diffuse O2, 2) it is highly biocompatible due to use of clinically proven Parylene material as well as its flexible mesh structure, and 3) it is a self-sustaining system that transports O2 from the ambient air via diffusion potential. These features will provide a physiological O2 environment for the graft and ensure safety in clinical applications. Our microdevice may serve as: 1) a platform for in vivo characterization studies using stem cell-derived beta cells, and 2) a clinical platform for shifting beta-cell replacement therapy from the current liver site into the SC site. To provide proof of concept, we will complete the following Aims: Optimization of the microdevice using rat islets in a diabetic rat model (Aim 1) and Validation of the microdevice using cadaveric human islets in an immunodeficient mouse model (Aim 2). In Aim 1, use of a well-established syngeneic rat SC-islet transplantation model will allow us to focus on the fabrication and oxygenation aspects of the device without immunoreaction bias in allogeneic/xenogeneic transplantations. In Aim 2, validating the microdevice in the SC site of immunodeficient mice using human islets from cadaveric donors will allow us bridge to subsequent future testing of human stem cell-derived beta cells. Our proposal is well-aligned with the goal of the HIRN Consortium on Human Islet Biomimetics to combine advances in beta cell and stem cell biology with tissue engineering technologies to develop microdevices. We expect successful completion of the proposed project to yield a novel microdevice that will ultimately improve cell replacement therapy for patients with T1D.
Publications
- Oxygen dynamics and delivery strategies to enhance beta cell replacement therapy
- Physiomimetic Fluidic Culture Platform on Microwell-Patterned Porous Collagen Scaffold for Human Pancreatic Islets
- A novel approach to determine the critical survival threshold of cellular oxygen within spheroids via integrating live/dead cell imaging with oxygen modeling
- Micropyramid-patterned, oxygen-permeable bottomed dish for high density culture of pancreatic islets
- Microwell culture platform maintains viability and mass of human pancreatic islets

