Identification of Novel Regulatory Mechanisms Driving Human Beta-Cell Maturation and Function
Contact PI: Romina Bevacqua, PhD, Mount Sinai School of Medicine (R03 DK138492)
Start Date: November 15, 2023
*Moved from CHIB to CMAD on December 1, 2024
NIH HIRN Gateway Investigator Award Recipient
Abstract
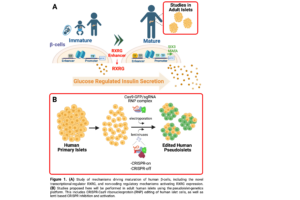
Type 1 Diabetes mellitus (T1D) is an autoimmune disease targeting pancreatic β-cells that affects over 8 million people worldwide. Standard treatment for T1D is limited to administration of exogenous insulin, which can result in hypoglycemic episodes, and fails to fully prevent micro- and macro- vascular complications. Thus, deriving glucose-responsive insulin-producing β-cells from renewable sources is an index goal of regenerative medicine. Yet, current human stem cell-derived β-cells produce insulin at lower levels than native β-cells and fail to recapitulate regulated insulin secretion typical of mature adult islet β-cells. This limits their therapeutic potential and reflects a significant gap in our knowledge regarding mechanisms regulating human islet maturation. Exciting recent studies identified human-specific age-dependent gene expression changes, including novel transcription factors, such as RXRG, not expressed in mouse or immature human β-like cells, that might control functional maturation of human islet cells. Our Preliminary Data shows that RXRG loss in human adult β-cells results in impaired glucose-stimulated insulin secretion. Recent studies also showed that gradual turnover of enhancers drives lineage progression and maturation. We have found a presumptive enhancer regulatory element of RXRG that becomes differentially accessible with age in adult β-cells compared to immature β-cells and a-cells, as measured by H3K27Ac occupancy. Our objective here is to discover mechanisms that induce maturation of human β-cells, using two approaches. In Aim 1, we will investigate the regulatory roles of the novel transcriptional regulator of β-cell maturation RXRG. This will involve knock down of RXRG, and then β-cell specific RNA-seq and CUT&RUN to identify RXRG downstream targets. In addition, we will activate RXRG endogenous promoter using the CRISPRa system and evaluate chromatin accessibility changes induced by activation of this maturation-required transcription factor. In Aim 2, we will study the non-coding regulatory mechanisms activating RXRG expression to induce β-cell maturation. Since these mechanisms do not occur in mouse or stem cell-derived human β-cells, and are specific to adult β-cells, the studies we propose will be performed in adult human islets using the pseudoislet-genetics platform Dr. Bevacqua recently developed. Here, we will use novel technological advancements, including CRISPR-Cas9 ribonucleoprotein (RNP) editing of human islet cells, to delete the presumptive enhancer element of RXRG and evaluate its role in regulating RXRG expression. Finally, we will activate RXRG expression in juvenile islets using CRISPRa to activate RXRG promoter, enhancer or both, and evaluate if RXRG expression enhances coordinated glucose-dependent insulin secretion in adulthood. Altogether, findings from this proposal will uncover novel pathways leading to β-cell maturation, that could be exploited towards efforts with β-cells from renewable sources. Equally importantly, these studies provide a novel toolkit for genetically manipulating previously “unstudiable” or inaccessible non-replicating mature and immature β-cells.