Survival and Potential of Insulin-deficient Beta Cells in Type 1 Diabetes
Contact PI: Desmond Schatz, MD, University of Florida (U01 DK135001)
Martha Campbell-Thompson, PhD, University of Florida
Clive Wasserfall, PhD, University of Florida
Yuval Dor, PhD, Hebrew University
Start Date: September 20, 2022
Abstract
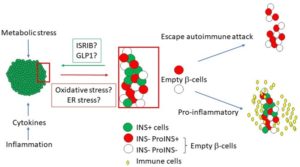
Recent evidence put forth by our group and others suggests that the pancreas of patients with recent-onset type 1 diabetes (T1D) contains a significant number of “empty” beta cells that have lost the ability to secrete mature insulin but retained other hallmark features such as proinsulin expression. The mechanisms that underlie formation of such cells and their fate remain poorly understood. We hypothesize that empty beta cells, which are invisible to standard insulin immunostaining and potentially also to the immune system, play important roles in the pathophysiology of T1D while holding therapeutic potential for reversion of diabetes. We propose in depth experiments to characterize empty beta cells, using MACSima ultra high-content immunofluorescence imaging to assess in situ expression of 78 immune and pancreas cell markers, including vascular and lymphatic annotation with signatures of inflammation, extravasation/trafficking, and immune cell residency in autoantibody positive (AAb+) and T1D donors as compared to donors with type 2 diabetes (T2D) and non-diabetic controls available through the Network for Pancreatic Organ donors with Diabetes (nPOD) repository. We will compare insulin containing versus insulin negative islets, within and across donors, to identify empty beta cells and determine how they correlate with islet, acinar, and immune cell phenotypes. These data will serve as a template for a serial section to undergo laser capture microdissection (LCM) of insulin containing islets, insulin negative islets, and acinar tissue regions, which will be subjected to bulk RNAseq as well as our novel method for quantifying beta cells based on DNA methylation patterns. In addition, we will use a novel mouse model (beta cell-specific, tamoxifen-inducible Adar1-mutant) and cultured human pancreas slices to functionally interrogate molecular pathways underlying the formation of empty beta cells. Specifically, we propose to leverage these two model systems to test therapeutic candidates— including an incretin mimetic (GLP1), an endoplasmic reticulum (ER) stress inhibitor (ISRIB), and multiple biologics targeting specific immune subsets— for their ability to modulate empty beta cells, their insulin content and insulin secretion. We will then correlate these functional data with molecular and cellular features of hormone negative islet cells via MACSima, RNAseq, and DNA methylation. These studies are expected to yield insights into a fundamental yet little understood process taking place in human T1D. We anticipate that empty beta cells can be re-functionalized, paving the way for therapeutic development to restore endogenous beta cell function in T1D. Ultimately, when combined with effective interventions to constrain autoimmunity, it is our hope that the metabolic modalities explored here could provide a means to reduce insulin requirements or even achieve insulin independence after T1D onset, dramatically improving longevity and quality of life for these patients.
Publications
- Beta cell dysfunction occurs independently of insulitis in type 1 diabetes pathogenesis
- Loss of Insulin-Positive Cell Clusters Precedes the Decrease in Islet Frequency and β-Cell Area in Type 1 Diabetes
- 3D imaging of human pancreas suggests islet sizeand endocrine composition influence their loss intype 1 diabetes
- 3D imaging of human pancreas suggests islet size and endocrine composition influence their loss in type 1 diabetes
- BETA CELL DYSFUNCTION OCCURS INDEPENDENTLY OF INSULITIS IN TYPE 1 DIABETES PATHOGENESIS
- Senescence of human pancreatic beta cells enhances functional maturation through chromatin reorganization and promotes interferon responsiveness
- Disrupted RNA editing in beta cells mimics early-stage type 1 diabetes

