Using ex vivo, in vivo Models and Patient Mutations to Interrogate Pancreatic Exocrine-Endocrine Cross Talk
Contact PI: Rohit Kulkarni, PhD, Joslin Diabetes Center (U01 DK135095)
Saumya Das, MD, PhD, Massachusetts General Hospital
Juan Dominguez-Bendala, PhD, University of Miami
Start Date: September 30, 2022
Abstract
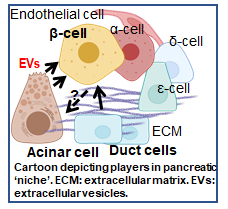
Type 1 diabetes and its metabolic consequences continue to be among the most significant biomedical challenges in the US and worldwide today. In addition to morbidities specifically related to diabetes the disease is associated with complications such as renal failure, lipid disorders, cardiovascular disease and cancer and is a major cause of death worldwide. Thus there is an urgent need for a better understanding of the pathogenesis that promotes the loss of insulin-secreting beta cells to plan better therapeutics to combat the disease. Several studies have argued that diverse cell types that make up the pancreatic niche contribute to the pathogenesis of the disease process. Thus a better understanding of the inter-cellular communication between major cells such as the acinar and duct cells and islet cells are warranted. We have studied the role of the mutant carboxy ester lipase (CEL) gene, expressed in acinar cells, on its ability to be taken up by beta cells and cause defects in its function and growth. The goal of this proposal is to discover the changes that occur when secretome from acinar versus duct cells are incubated with human islet and β-cells. The data from these studies will generate new hypothesis for testing that will allow a deeper interrogation of the cross-talk between human acinar, duct and islet cells. We will address the following Aims in this proposal: Aim 1) Determine the ability of human acinar versus human duct cells to directly impact islet cell function. We will isolate and characterize EVs, from human induced pluripotent stem (hIPS) cell derived acinar- versus duct-lineage committed organoids, and incubate them with human islet/β-cells and human pancreas slices to directly examine the consequences on islet cell biology. We will also examine the EV cargo with a particular focus on fragments derived from transfer RNAs. Aim 2) We will interrogate the ability of EVs, derived from acinar-derived organoids generated from the hiPS cells from MODY8 patients, to directly regulate human islet-β-cell biology. The use of human organoids and human pancreas slices will provide translational relevance of our studies. The results and datasets obtained from these experiments will complement the efforts of the Human Islet Research Network and provide novel resources to be shared with the larger scientific community.
Publications
- Searching for protein partners of short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD) reveals keratin 8 as a novel candidate for interaction in pancreatic β-cells
- Everything You Wanted to Know About Pancreatic Ducts But Didn’t Know Where to Look
- Stress and human health in diabetes: A report from the 19(th) Chicago Biomedical Consortium symposium
- Integrated Physiology of the Exocrine and Endocrine Compartments in Pancreatic Diseases: Workshop Proceedings

