The Role of Senescent Beta Cells in Type 1 Diabetes and Type 2 Diabetes
Contact PI: Klaus Kaestner, PhD, University of Pennsylvania (U01 DK134995)
Benjamin Glaser, MD, Hadassah Medical Center
Dana Avrahami-Tzfati, PhD, Hadassah Medical Center
Al Powers, MD, Vanderbilt University
Start Date: September 20, 2022
Abstract
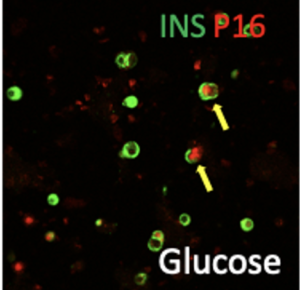
The role of senescent beta cells in T1D and T2D Abstract Recent studies including our own suggest a marked increase in β-cells expressing key components of cellular senescence in islets from Type 1 diabetes (T1D) and Type 2 diabetic (T2D) patients, implicating β-cell senescence as a critical contributor to islet dysfunction. Recently, it was reported that ablation of senescent cells by non-specific senolysis in mouse models of T1D and T2D improved diseease outcome. However, these studies did not determine which senescent cell type was relevant to the beneficial effect, nor did they address to what extent senescence occurs in the human endocrine pancreas before and during the development of T1D and T2D. To close this knowledge gap, in Specific Aim 1 we will determine the prevalence, transcription signatures and epigenomic landscapes of β-cell senescence in T1D, pre-T1D and T2D donors using immunostaining, imaging mass cytometry, single cell RNAseq, single cell ATACseq, DNA methylome determination and Cut-and-Tag analysis for key histone marks. In addition, we will evaluate the hypothesis that irreparable damage to telomeres drives senescence in β-cells, a possible scenario that could provide a mechanism for senescence to occur in islet cells of diabetic patients. In Specific Aim 2, we will test whether metabolic and/or inflammatory stressors drive senescence in human β-cells and determine the effect of senescence on β-cell function using scRNAseq and secretome analysis. We will evaluate if induction of senescence and the senescence-associated secretory phenotype (SASP) in human islet cells is p16 dependent, employing the pseudo-islets approach and using our hyperglycemic xeno-transplantation model to assess the direct effect of senolytics on human islet function. In Specific Aim 3, we will employ a novel transgenic mouse, the ‘SenKiller’ model, to enable cell-type specific and inducible ablation of senescent cells in any lineage including β-cells. Using this mouse model in combination with the appropriate β-cell specific Cre driver, we will provide a definitive answer to the question if senescent β-cells are critical in the development of glucose intolerance in models of T2D and islet autoimmunity in models of T1D. Together, this proposal will determine the occurrence of senescence among islet cells from T1D and T2D donors using large cohorts and multiple experimental modalities, explore the natural drivers of senescence and consequences to islet function as well as secretion of pro-inflammatory substances, and employ novel mouse models to unequivocally determine if elimination of senescent b-cells impacts diabetes progression in mouse models of T1D and T2D. The data generated here will address burning questions in the field, namely, is senescence increased in islets from diabetic patients, and are these cells important in the overall pathophysiology of T1D and T2D. These critical questions will have therapeutic relevance regarding the potential efficacy of targeting senescent β-cells with senolytic therapies.
Publications
- Epigenetic adaptation of beta cells across lifespan and disease: age-related demethylation is advanced in type 2 diabetes
- G6PC2 controls glucagon secretion by defining the set point for glucose in pancreatic α cells
- The IsletTester mouse: an immunodeficient model with stable hyperglycemia for the study of human islets
- Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression
- Senescence of human pancreatic beta cells enhances functional maturation through chromatin reorganization and promotes interferon responsiveness

