Linking Human Islet Structural Heterogeneity to Beta Cell State
Contact PI: Julie Sneddon, PhD, University of California, San Francisco (U01 DK135019)
Zev Jordan Gartner, PhD, University of California, San Francisco
Start Date: September 20, 2022
Abstract
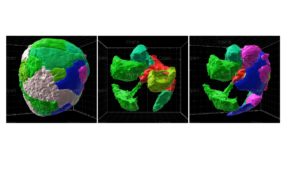
The detailed structure of the beta cell niche, and that of the islet in general, remains poorly understood; this is particularly the case for human islets. Islet structure appears heterogeneous across the pancreas, and whether conserved structural features exist among islets is unknown. A detailed understanding of the organizational principles of islets would advance our ability both to reconstitute stem-cell derived islets as a cure for type 1 diabetes (T1D) and to block the progression of events that lead to the loss of beta cells during the progression of diabetes. Therefore, the goal of this proposal is to identify and experimentally validate the critical organizational principles of the islet in general and the beta cell niche in particular.
Towards this goal, we have developed a custom, semi-automated, 3D imaging and analysis pipeline that permits quantification of the statistical properties of the beta cell niche at sub-micron resolution and across hundreds of individual beta cells. Our preliminary analyses of healthy mouse and human islets have revealed that (1) in both species, beta and delta cells maintain at least one physical contact with vascular basement membrane, but in human islets the alpha cells do not, and (2) reconstituting stem cell-derived beta cells into pseudo-islets in a manner that maximizes their contact with basement membrane improves their response to glucose by at least two-fold in vitro and further extends their functionality in vivo. We hypothesize that beta cell contact with basement membrane is a conserved element of islet structure that must be incorporated into engineered islets to optimize beta cell function.
Building on these preliminary findings, we now aim to dramatically expand this analysis across tens of thousands of individual cells in human and mouse islets, incorporating all endocrine cell types along with immune cells, vascular cells, and nerves. This will result in the first quantitative assessment of the endocrine cell structural niche that acknowledges the structural heterogeneity of islets and aims to identify conserved structural motifs. Taken together, our study will provide the first quantitative structural blueprint for the pancreatic islet, and will identify features of the beta cell niche that are conserved and divergent across humans and mice. This work will contribute towards future development of strategies for reconstituting more functional human tissues from stem cells that use a structural blueprint to guide tissue engineering.